Read more about clinical studies here.
Learn more about other sites with information about clinical studies here.
A glossary of terms related to clinical trials can be viewed here.
Clinical trials are often thought of as a treatment option of last resort for individuals who are very ill or facing a life-threatening disease. But clinical trials can offer different opportunities to many different types of individuals. Clinical trials can offer hope to people who may not be responding well to currently available therapies, and many trials actually seek out healthy volunteers to participate in research studies.

In the field of venous thromboembolism, or in clinical trials studying deep vein thrombosis (DVT) and pulmonary embolism (PE), there are literally hundreds of studies taking place throughout the United States and around the globe.
If you’re interested in participating in a clinical trial, your first step should be to learn more about clinical trials, including how they are conducted, how they enroll and work with patients, and what you can expect if you find yourself part of a clinical trial.
Below we address some basic questions about clinical trials, and then provide direction and resources to help you explore existing clinical trials taking place today relative to DVT and PE.
Clinical trials are used in medical research to help evaluate and determine the safety and effectiveness of new drugs treatments, and to compare different treatments or approaches to treatment. Some clinical trials also explore the use of new diagnostic tools and medical devices, while others might look at the development or progression of disease in certain patient populations.
Carefully designed clinical trials are the safest and quickest way to find treatments that work. Ideas for clinical trials usually come from researchers who first test new therapies or procedures in the laboratory and get promising results prior to planning clinical trials or research that will involve human subjects or patients. New therapies are tested on people only after laboratory and animal studies show that they are generally safe and may offer promising results.
Clinical trials are sponsored by by government agencies, such as the National Institutes of Health, pharmaceutical companies, healthcare organizations, individual physicians or researchers, and companies that research and develop other medical products, including diagnostic tests, medical devices, and medical or surgical equipment.
Clinical trials can take place in a number of locations, including doctors’ offices, community health centers or clinics, hospitals, and universities.
More detailed information about clinical trials can be found here.
The design of all clinical trials is based on a set of rules called a protocol. A protocol defines the types of people who can participate in the trial, the type of tests that will be performed, a description of the medication(s)
 and their doses, other procedures involved in the study, and the projected timeline for these activities.
and their doses, other procedures involved in the study, and the projected timeline for these activities.
Patients who are involved in clinical trials are usually seen fairly regularly by research staff to monitor their health and conduct evaluations of the effects of the medication(s) being studied in terms of safety and effectiveness.
There are four phases of clinical trials involving experimental drugs:
Phase I clinical trials involve a small group of people to evaluate a new drug’s safety and to determine a safe dosage range and potential side effects.
Phase II clinical trials involve a larger group of people to further evaluate if the new drug might be effective and to continue to confirm that it is safe for use in human subjects.
Phase III clinical trials expand research of the new drug or therapy among a larger group of people to confirm its effectiveness, monitor side effects, compare it to commonly used treatments, and collect information that will allow the drug or treatment to be used safely.
Phase IV studies, also called post-marketing surveillance studies, take place after the drug or treatment has been approved by the U.S. Food and Drug Administration for marketing or commercial use. Phase IV studies collect information about the effects of a new drug in a real world setting in various populations and can help identify long-term side effects.

All clinical trials have guidelines or criteria that identify who can and cannot participate in a research program.
There are inclusion criteria that state clearly who the study subjects are intended to be, while exclusion criteria indicate who should not be included. These criteria are not meant to reject people personally. Rather, the criteria are used to identify appropriate participants and to make sure they are kept safe and also enable researchers to get the information about the medication they plan to study.
These guidelines may be based on an assortment of factors, including age, type of disease, medical history, and current medical condition.
Before you join a clinical trial, you must qualify for the study. Some research studies seek volunteers with illnesses or conditions to be studied in the clinical trial, while others need healthy volunteers.
There are strict government guidelines in place to ensure that people who participate in clinical trials remain safe. All planned clinical trials that are designed for implementation in the U.S. must be reviewed, approved, and monitored by an institutional review board (IRB) made up of physicians, patient advocates, researchers, and other stakeholders who can help ensure that the study is ethical and that the rights and safety of the participants are protected. Federal regulations require that all institutions that conduct medical research involving human subjects have an IRB that initially approves and periodically reviews the research.

Another crucial aspect of clinical trials that helps to safeguard patients involves informed consent, or the process a patient goes through to learn about a clinical trial before they decide whether or not to participate.
Before consenting to participate in a clinical trial, an informed consent document should explain the goals of the research, the procedures and timeline, the potential risks and benefits, other treatment options that might be available, and the patient’s right to leave the trial at any time. Participating in a clinical trial is a major decision, and you should ask your doctor or the research staff any questions you have before signing the consent form.
However, it’s important to understand that informed consent is a process that continues after you sign the form. Informed consent continues throughout the duration of the study, and you should feel free to ask the research staff questions before, during, and after the study.
If you are interested in participating in a clinical trial, talk to your doctor to determine if they are familiar with any trials that might be a good match for you. Your doctor also can help you determine if studies you might find on your own are a good opportunity.
There also are tools available online to facilitate your research. One of the most popular and easy-to-use tools is available at clinicaltrials.gov. You can access thousands of clinical trials, including many involving DVT/PE diagnosis, treatment, and longer-term management.
To find a specific study or to narrow your search of studies of interest to you, you can enter specific search terms at the top of the clinicaltrials.gov home page.
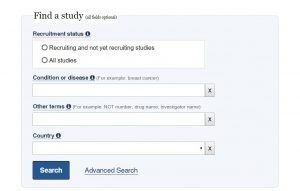 As shown to the right, you simply enter your search terms into the data fields, then click “search.” You will then be taken to a page that shows the studies that correspond with your search terms, if they exist.
As shown to the right, you simply enter your search terms into the data fields, then click “search.” You will then be taken to a page that shows the studies that correspond with your search terms, if they exist.
You can access specific instructions for how to effectively search www.clinicaltrials.gov here, and additional helpful information for patients and families can be viewed here.
Below are several potential search terms that, if you click on them, will link you to current studies in those areas as they appear on clinicaltrials.gov:
New studies are published on the website and existing studies are updated daily. To ensure that you are accessing the most up-to-date information, we encourage you to take some time to familiarize yourself with how searches are done effectively on the site, and then do your search in real time to ensure you are seeing the latest search results.
As you are exploring different clinical trials, you can download and print the list of questions in the link below to discuss with your doctor or your clinical trials research team.
Clinical-trials-questions-to-consider