COVID-19 and Blood Clotting
COVID-19 Vaccines
 The first two vaccines for the prevention of COVID-19 were granted emergency use authorization by the United States Food & Drug Administration (FDA) in December 2020, and administration of these new vaccines (Pfizer and Moderna) began across the country several days later in mid-December 2020. An additional vaccine received EUA from the FDA in February 2021, and administration of this vaccine (Johnson & Johnson) began in the U.S. shortly thereafter.
The first two vaccines for the prevention of COVID-19 were granted emergency use authorization by the United States Food & Drug Administration (FDA) in December 2020, and administration of these new vaccines (Pfizer and Moderna) began across the country several days later in mid-December 2020. An additional vaccine received EUA from the FDA in February 2021, and administration of this vaccine (Johnson & Johnson) began in the U.S. shortly thereafter.
As the access to these COVID-19 vaccines expands, NBCA and its Medical & Scientific Advisory Board will monitor information that may be important for people affected by clots and clotting disorders, as well as those who are prescribed anticoagulation or “blood thinning” therapies.
Studies of the new vaccines against COVID-19 show that they are safe and effective and medical experts are recommending that people get vaccinated as soon as they can to prevent infection with the virus that causes COVID-19. People should speak with their healthcare providers about any questions they have about COVID-19 and the new vaccines being used to prevent infection with this highly contagious and very serious infectious disease.
Visit CDC’s website for more about COVID-19 vaccines and to review the most frequently asked questions: Vaccine FAQs.
Click on the links below to learn more about:
- Risk of Venous Thromboembolism After Covid-19 Vaccination
- Vaccine Information Specific to the Clotting Community
- Getting Your Vaccine
- Vaccination and the Variants of the Virus that Causes COVID-19
- News updates about the Johnson & Johnson Vaccine
- News updates about the Astra Zeneca Vaccine
COVID-19 Vaccine Boosters
COVID-19 vaccination is recommended for everyone aged 5 years and older in the United States for the prevention of COVID-19. Vaccines are effective in preventing serious outcomes of COVID-19, including severe disease, hospitalization, and death.
Three COVID-19 vaccines are approved or authorized by the Food and Drug Administration:
- Pfizer-BioNTech COVID-19 Vaccine
- Moderna COVID-19 Vaccine
- Johnson & Johnson (Janssen) COVID-19 Vaccine
As a booster dose, you may receive any of the COVID-19 vaccines authorized in the United States and are not limited to staying with the same brand as your original vaccination.
COVID-19 and Blood Clotting: Frequently Asked Questions
 Throughout the COVID-19 pandemic, NBCA has received numerous questions about the potential for clotting associated with COVID-19, as well as the use of the new COVID-19 vaccines.
Throughout the COVID-19 pandemic, NBCA has received numerous questions about the potential for clotting associated with COVID-19, as well as the use of the new COVID-19 vaccines.
While more information remains to be known and confirmed, we have compiled a list of Frequently Asked Questions (FAQs) to share the most current information available.
We will continue to update this resource as more information is made available, and your healthcare provider also may be able to help address your questions and share other resources.
CLICK THE FOLLOWING LINK TO ACCESS OUR DEDICATED WEB PAGE OF FAQS:
TO DOWNLOAD A PDF VERSION OF THESE FAQS, CLICK HERE:
TO DOWNLOAD A PDF VERSION OF THESE FAQS TRANSLATED FOR SPANISH-SPEAKING INDIVIDUALS CLICK HERE:
PARA DESCARGAR UNA VERSIÓN DE ESTAS PREGUNTAS FRECUENTES EN FORMATO PDF PARA PERSONAS DE HABLA HISPANA, HAGA CLIC AQUÍ:
TO DOWNLOAD A PDF VERSION OF THESE FAQS TRANSLATED FOR INDIVIDUALS WHO SPEAK MANDARIN (SIMPLIFIED CHINESE), PLEASE CLICK HERE:
如需下载为讲西班牙语的人翻译的这些常见问题解答的 PDF 版本,请点击这里:
NBCA Coronavirus Communications: COVID-19 and Blood Clotting
 Click on the links below to read more information about COVID-19 and blood clots shared by NBCA:
Click on the links below to read more information about COVID-19 and blood clots shared by NBCA:
- Risk of blood clots more than 3-fold higher in hospitalized adults with COVID-19
- CDC Grant Supports National Blood Clot Alliance COVID-19 Research
- NBCA Statement re: ISTH Consensus Document Concerning COVID-19 and VTE
- Preventing Hospital-Related Blood Clots
- Managing Stress and Anxiety During the Coronavirus Pandemic
- INR Testing During the COVID-19 Pandemic
Clinical Trials
NBCA is committed to providing reliable, evidence-based, and impartial information to the clotting and clotting disorders community to help members of our community make the most informed decisions in consultation with their healthcare providers. COVID-19 has affected millions of people worldwide. The pandemic has devastated our country, communities, friends, and families in ways that will be felt for generations. It has also brought dramatic changes in science and medicine and how healthcare is approached. Clinical trials and vaccines are critical to saving lives and the future health of each of us. NBCA is pleased to provide you with evidence-based, unbiased information about possible participation in clinical trials and also COVID-19 vaccines for your discussion with your healthcare provider.
Global Survey Documents Treatment Patterns to Prevent Blood Clots among Hospitalized Individuals with COVID-19
A group of clinicians and researchers – led by Rachel Rosovsky, MD, a hematologist at Massachusetts General Hospital, an Assistant Professor at Harvard Medical School, and a member of NBCA’s Medical & Scientific Advisory Board – recently conducted a global survey among more than 500 physicians from 41 different countries to document how healthcare providers are preventing, diagnosing, and treating blood clots in people with COVID-19. Below (right) is an infographic that visually depicts key findings from this survey. Information from this survey, published in the medical journal Research and Practice in Thrombosis and Haemostasis, shows:
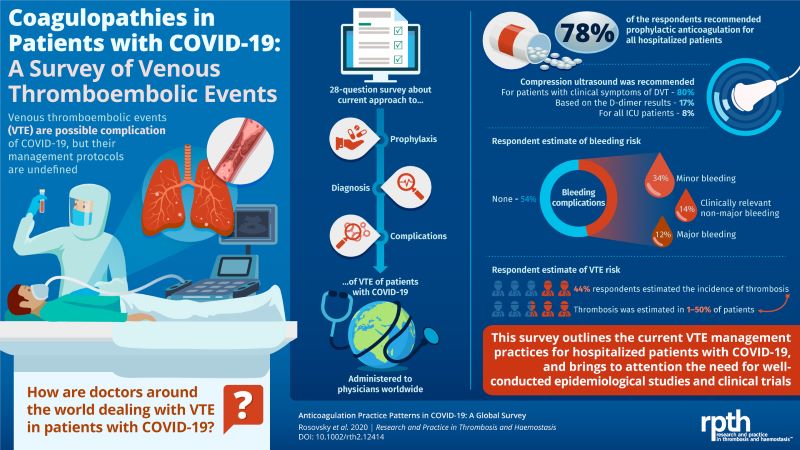 The majority (78 percent) of the survey respondents reported that they recommend prophylactic (intended to prevent disease) anticoagulation therapy to help prevent blood clots from forming in individuals hospitalized with COVID-19. There was a wide variation in practice when respondents were asked in what situations they would increase the dose of anticoagulation.
The majority (78 percent) of the survey respondents reported that they recommend prophylactic (intended to prevent disease) anticoagulation therapy to help prevent blood clots from forming in individuals hospitalized with COVID-19. There was a wide variation in practice when respondents were asked in what situations they would increase the dose of anticoagulation.- Bleeding, a potential side effect of any anticoagulation therapy, was addressed by most (73 percent, n=377) of the survey respondents, with more than half (54 percent) of this group reporting that they saw no bleeding, about one-third (34 percent) reporting minor bleeding, and smaller numbers of respondents reporting clinically relevant bleeding (14 percent) or major or potentially life-threatening bleeding (12 percent).
- Most (75 percent, n=391) of the survey participants responded to questions about using compression ultrasound (a medical imaging technique that involves the placement on the skin of a probe that uses soundwaves to construct an image of tissue beneath the skin to determine if a clot may be present) to diagnose a deep vein thrombosis (DVT, or blood clot in the leg or arm) among people affected by COVID-19. Specifically:
- 80 percent of this group reported that they obtained compression ultrasound DVT symptoms only if a patient had symptoms of a DVT
- 17 percent recommend compressional ultrasound if a patient had an elevated D-dimer (a blood test that may indicate if a person has elevated levels of the D-dimer protein fragment that remains after a clot forms)
- 8 percent recommended compression ultrasound in all hospitalized patients who were critically ill and in the intensive care unit (ICU)
- A portion (44 percent) of the survey respondents shared their estimates for the potential incidence of clotting among patients hospitalized for COVID-19, with estimates ranging from as low as 1 percent up to 50 percent, with higher numbers in ICU than among all hospitalized patients.
The group of medical experts who conducted this survey stress the urgent need for both well-designed studies to help the medical community understand the incidence and risk factors for blood clots among people affected by COVID-19, as well as randomized clinical trials to address the use of anticoagulation therapies among these same individuals.
Healthcare professionals (HCPs) can track global updates relative to COVID-19, using the interactive map created and maintained by Johns Hopkins University here: Johns Hopkins COVID-19 Dashboard. (Reference: Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real-time. Lancet Infect Dis; published online Feb 19, 2020.)
In addition, HCPs can review guidance from the Anticoagulation Forum here and the American Society of Hematology here.
Risk Factors, Signs and Symptoms
Now more than ever, it’s crucial to understand the risk factors for blood clots, and also the signs and symptoms that might signal you need to seek medical attention.

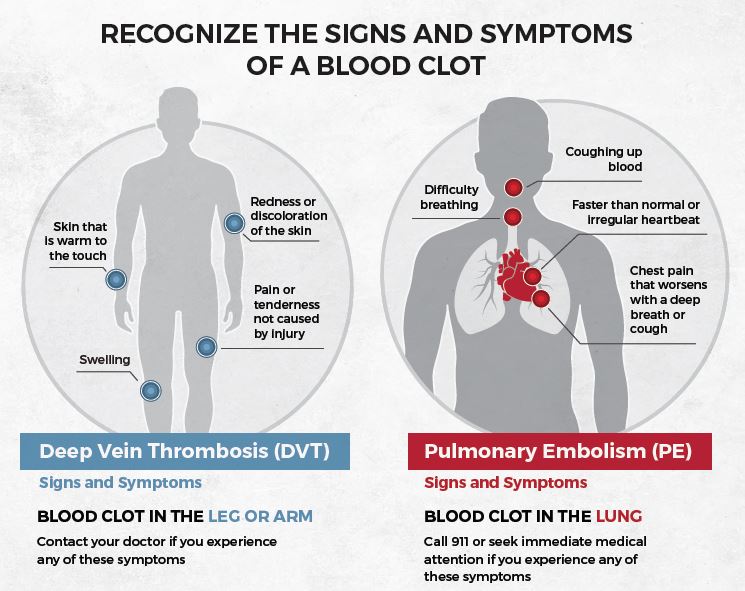
Learn more about blood clot risk factors here: Risk Factors
Learn more about blood clot signs and symptoms here: Signs and Symptoms
Episode 1: Covid-19 Treatment Options for Blood Clot Patients on Anticoagulants
Learn more about Covid-19 treatment options for blood clot patients on anticoagulants. This mini PEP Talk session is joined by special guest and NBCA Medical and Scientific Advisory Board Member Dr. Caroline Cromwell, a hematologist and the Director of Thrombosis Services at Mt. Sinai Medical Center in New York City.
Healthcare Professionals: Our Best Resources
 The information provided here is not intended to serve as a substitute for professional medical advice. Please talk to your physician or medical team about any questions you have concerning your health or COVID-19 and clotting.
The information provided here is not intended to serve as a substitute for professional medical advice. Please talk to your physician or medical team about any questions you have concerning your health or COVID-19 and clotting.
If you need help in finding a second opinion or medical expert in your local community, you can use one of the search tools at the following link to identify medical experts in your local community: Find a Doctor.
